Research Summary
High pressure and high temperature chemistry

The group focuses on understanding structure-property relationship of functional materials under extreme conditions.
The overall objectives of the work of Chuvashova Lab in materials science are to
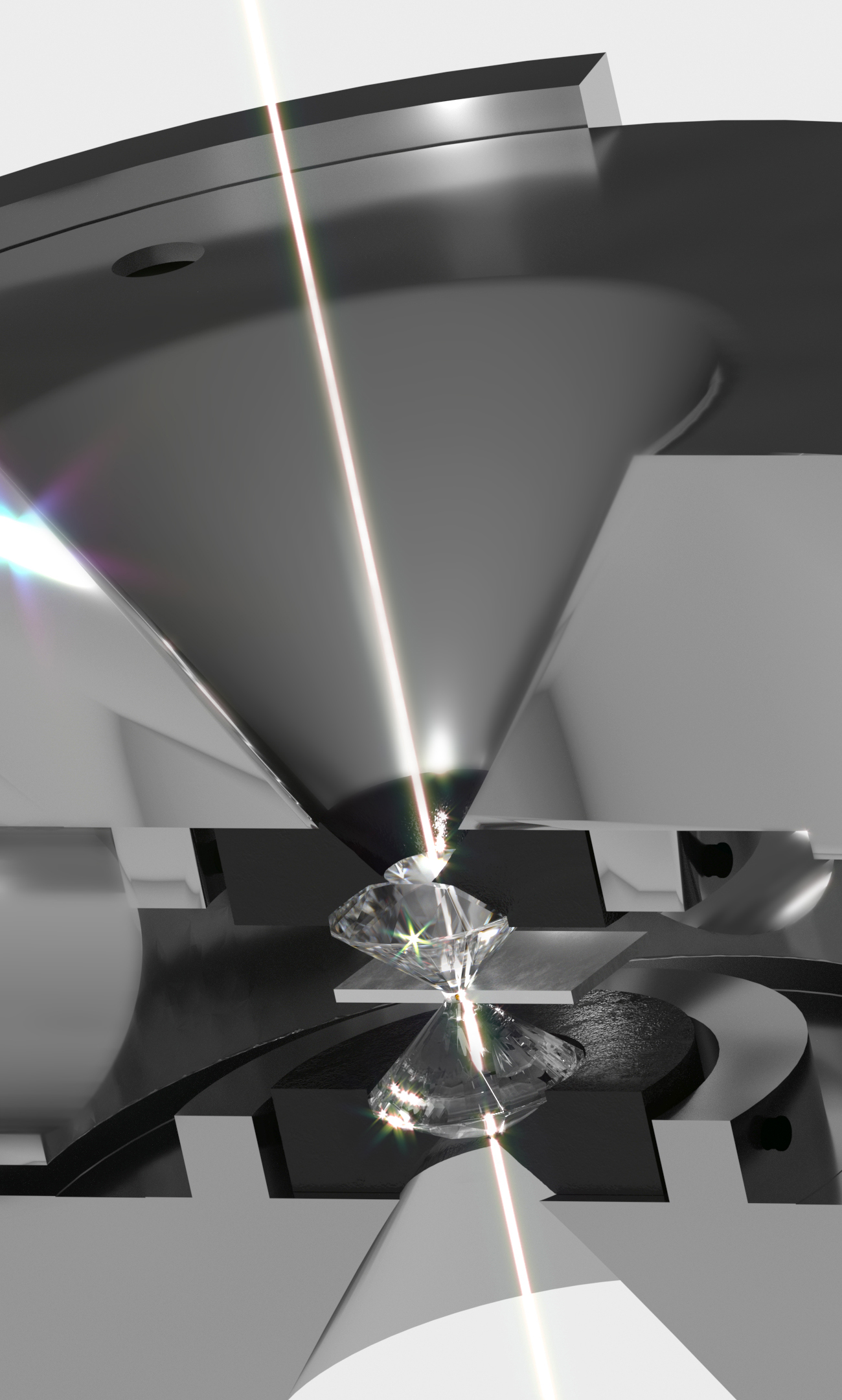
By investigating the HPHT and HPLT behaviors of elemental materials and compounds, my research sheds light on bonding evolution, phase transformations, and the development of PT-phase diagrams of fundamental importance in materials synthesis and their applications. In particular, my state-of-the-art research focuses on developing new materials that are as strong as steel, but of lower density than water, by combining various methods of synthesis at extreme conditions using large-volume-press techniques, diamond anvil cells (DACs), and double-sided laser heating that can generate temperatures above 1200 K. This work will additionally entail honing existing synthetic and analytical methods to produce the kinds of chemical reactions conducted at extreme conditions of HPHT and HPLT that can trigger structural, electronic, and magnetic changes in matter and exhibit behaviors that are distinct from that at ambient conditions.
Applications of this research include new energy-efficient and environment-friendly materials, and new materials for possible use in aircraft production and space exploration.
The overall objectives of the work of Chuvashova Lab in materials science are to
- Elucidate the behavior of materials exposed to extreme conditions, notably high pressures (HP) and high and temperatures (HT);
- Create new functional materials with interesting and useful properties;
- Develop new designs for experimental approaches.
By investigating the HPHT and HPLT behaviors of elemental materials and compounds, my research sheds light on bonding evolution, phase transformations, and the development of PT-phase diagrams of fundamental importance in materials synthesis and their applications. In particular, my state-of-the-art research focuses on developing new materials that are as strong as steel, but of lower density than water, by combining various methods of synthesis at extreme conditions using large-volume-press techniques, diamond anvil cells (DACs), and double-sided laser heating that can generate temperatures above 1200 K. This work will additionally entail honing existing synthetic and analytical methods to produce the kinds of chemical reactions conducted at extreme conditions of HPHT and HPLT that can trigger structural, electronic, and magnetic changes in matter and exhibit behaviors that are distinct from that at ambient conditions.
Applications of this research include new energy-efficient and environment-friendly materials, and new materials for possible use in aircraft production and space exploration.

Thermoelectric materials on the base of Rare-Earth Elements at Extreme Conditions

Novel materials can be synthesized under high pressure with enhanced properties, such as high energy density, superhardness, and high conductivity. Although materials synthesized under high-pressure conditions are not the most stable form at ambient conditions, they could be metastable upon pressure release due to a large energy barrier for the chemical transformation. This notion is in line with most previous high-pressure studies, where increased temperature is required to promote the synthesis of compounds at elevated pressures to overcome the reverse energy barrier between ambient and sought-after high-pressure stoichiometry.
One of the significant challenges in the field is to devise materials that will be metastable upon pressure release and to recover these high-pressure materials with alternate stoichiometry at ambient conditions for possible useful applications. A well-known elemental analogue is the mass production of synthetic diamonds under high-pressure conditions, with various practical applications such as machining and cutting tools. Although the stable form of carbon at ambient conditions is graphite, synthetic diamonds are metastable upon pressure release due to the large energy barrier of the sp3 to sp2 conversion.
One of the significant challenges in the field is to devise materials that will be metastable upon pressure release and to recover these high-pressure materials with alternate stoichiometry at ambient conditions for possible useful applications. A well-known elemental analogue is the mass production of synthetic diamonds under high-pressure conditions, with various practical applications such as machining and cutting tools. Although the stable form of carbon at ambient conditions is graphite, synthetic diamonds are metastable upon pressure release due to the large energy barrier of the sp3 to sp2 conversion.

Applying high pressure to metal (primarily Rare-Earth Elements) borides will uncover chemical reactions not possible at ambient conditions and open the boundaries of new compounds of the metal-boron system, and illuminate reaction mechanisms and behavior, leading to the creation of materials with the desired chemistry and properties.
Materials with promising thermoelectric properties are desired for their use in emerging technologies and applications. Thermoelectric materials conduct electricity using a temperature difference in the material, and they can utilize waste heat to generate more electricity. Their function makes these materials sought out for usage in applications where heavy thermal waste is expected and where energy efficiency is critical. Despite its promise, thermoelectric materials typically have poor efficiency, limiting widespread usage of these materials. This limitation leads us to search for a class of material that overcomes this issue and can be used to address the technological needs. Rare-earth (RE) borides may be used as thermoelectric materials. Previously, RE borides were studied experimentally and computationally for properties like superconductivity, magnetic phase transitions, and hardness. RE borides can exist as either a lower or higher boride and have been observed to exist in several configurations: REB2, REB4, RE2B5, REB6, REB12, REB25, REB50, and REB66, this range of stoichiometries involves distinct structural boron arrangements and polyhedra that alter the properties of each compound. Consequently, this can influence the structure's overall stability at both elevated pressures and its path to ambient pressure.
The synthesis and investigation of metal borides under extreme conditions are of great interest for material science and technology, given the interesting properties of these compounds, namely superconductivity, low compressibility and high hardness, high melting points, good thermal, and chemical stability.
Main focus is on compounds that can be synthesized at low pressure and temperature aiming to achieve mass production of the material using thermodynamic conditions that can be reproduced (scaled up) by large volume presses.
Materials with promising thermoelectric properties are desired for their use in emerging technologies and applications. Thermoelectric materials conduct electricity using a temperature difference in the material, and they can utilize waste heat to generate more electricity. Their function makes these materials sought out for usage in applications where heavy thermal waste is expected and where energy efficiency is critical. Despite its promise, thermoelectric materials typically have poor efficiency, limiting widespread usage of these materials. This limitation leads us to search for a class of material that overcomes this issue and can be used to address the technological needs. Rare-earth (RE) borides may be used as thermoelectric materials. Previously, RE borides were studied experimentally and computationally for properties like superconductivity, magnetic phase transitions, and hardness. RE borides can exist as either a lower or higher boride and have been observed to exist in several configurations: REB2, REB4, RE2B5, REB6, REB12, REB25, REB50, and REB66, this range of stoichiometries involves distinct structural boron arrangements and polyhedra that alter the properties of each compound. Consequently, this can influence the structure's overall stability at both elevated pressures and its path to ambient pressure.
The synthesis and investigation of metal borides under extreme conditions are of great interest for material science and technology, given the interesting properties of these compounds, namely superconductivity, low compressibility and high hardness, high melting points, good thermal, and chemical stability.
Main focus is on compounds that can be synthesized at low pressure and temperature aiming to achieve mass production of the material using thermodynamic conditions that can be reproduced (scaled up) by large volume presses.
Novel solar energy materials at extreme conditions
This project is done in collaboration with Dr. George Nolas's group from the University of South Florida. It focuses on two compounds: Cu2ZnSnSe4 and GdTe1.8.
In exploring new materials for thermoelectric applications, it is sometimes of interest to investigate materials that show promise in other energy conversion technologies where, in many ways, similar materials criteria are of interest. The semiconductor Cu2ZnSnSe4 (CZTSe) is one example. Its constituents are made of earth-abundant, non-toxic materials, and the electrical properties can be modified by appropriate doping and/or variations in stoichiometry. In addition, the relative complexity of its crystal structure results in a low thermal conductivity. These factors result in good thermoelectric properties for CZTSe composites, i.e. with a coating
reported to prevent sublimation of selenium or high-pressure torsion treatment.
Structure differences created by the non-stoichiometry of components of GdTe1.8 lead to new magnetic and semiconducting properties. At ambient conditions, it has a narrow, highly conducting band gap which can be used for IR sensors, low-light solar cells, or quantum computing.
These materials have promising and exciting properties at ambient conditions. However, there are no studies of their behavior at elevated pressures and temperatures.
In exploring new materials for thermoelectric applications, it is sometimes of interest to investigate materials that show promise in other energy conversion technologies where, in many ways, similar materials criteria are of interest. The semiconductor Cu2ZnSnSe4 (CZTSe) is one example. Its constituents are made of earth-abundant, non-toxic materials, and the electrical properties can be modified by appropriate doping and/or variations in stoichiometry. In addition, the relative complexity of its crystal structure results in a low thermal conductivity. These factors result in good thermoelectric properties for CZTSe composites, i.e. with a coating
reported to prevent sublimation of selenium or high-pressure torsion treatment.
Structure differences created by the non-stoichiometry of components of GdTe1.8 lead to new magnetic and semiconducting properties. At ambient conditions, it has a narrow, highly conducting band gap which can be used for IR sensors, low-light solar cells, or quantum computing.
These materials have promising and exciting properties at ambient conditions. However, there are no studies of their behavior at elevated pressures and temperatures.
Non-linear optical materials

The project aims to create suitable crystals for different optical applications, in particular, nonlinear optical (NLO) materials. NLO can be used to generate new laser sources of frequencies that cannot be obtained directly from available lasers. We are interested in materials for deep UV lithography (DUV) and second-harmonical generators (SHG).
Borates have a great industrial and academic interest due to their unique physical and chemical properties. The main building blocks of borates are the anions: [BO2]-, [BO3]3-, and [BO4]5-, but only the RE borates with planar [BO3]3- groups have promising optical properties for the DUV and SHG applications. The main complication in creating RE borates crystals is synthesis: we need to precisely monitor the pressure and temperature to keep the anions in the right configuration. Therefore, we are trying to study the synthesis conditions.
Borates have a great industrial and academic interest due to their unique physical and chemical properties. The main building blocks of borates are the anions: [BO2]-, [BO3]3-, and [BO4]5-, but only the RE borates with planar [BO3]3- groups have promising optical properties for the DUV and SHG applications. The main complication in creating RE borates crystals is synthesis: we need to precisely monitor the pressure and temperature to keep the anions in the right configuration. Therefore, we are trying to study the synthesis conditions.
Nuclear materials
Proper management of nuclear waste is crucial for long-term sustainability, as it can pose a threat if not contained. To address this, materials science is playing a pivotal role in developing innovative solutions to ensure safe storage and disposal of nuclear waste.
Our interest in f-element borates is based on the potential formation of trivalent f-element borate-based complexes in nuclear defense waste, for example, at the Waste Isolation Pilot Plant. Lanthanides and actinides show a similar trend in behavior as the contraction across the series represents a total change of 0.15 Å. For lanthanides, the trivalent state is the most stable, however, for actinides only by americium, the trivalent state becomes the predominant. Curium, Americium, and Plutonium are the main components of the nuclear fuel cycle. Like CmIII, GdIII has a half-filled f shell with 7 unpaired electrons, which leads to its interesting magnetic properties. However, it’s known that CmIII has unique electronic properties that result in new borate structure formations in nuclear waste conditions. This difference and deep understanding of structural and behavioral differences under different conditions can lead to a new way of separating trivalent lanthanide fission products that act as neutron poisons for AmIII and CmIII.
Borates have complex structures, with boron atoms that can exist in both trigonal-planar (BO3) and tetrahedral (BO4) arrangements. These arrangements respond differently to subtle differences in chemical bonding, leading to varying structures. Furthermore, time, temperature, and pressure significantly influence borates' structural arrangements. High-pressure high-temperature synthesis provides a powerful method to create and analyze these borates, allowing greater control of sample composition and enabling the obtainment of metastable materials. The study also highlights the interest in combining the structural and chemical aspects of borate and perrhenate on trivalent f-elements, which has been shown to completely alter the structures, resulting in the creation of new types of structures.
Our interest in f-element borates is based on the potential formation of trivalent f-element borate-based complexes in nuclear defense waste, for example, at the Waste Isolation Pilot Plant. Lanthanides and actinides show a similar trend in behavior as the contraction across the series represents a total change of 0.15 Å. For lanthanides, the trivalent state is the most stable, however, for actinides only by americium, the trivalent state becomes the predominant. Curium, Americium, and Plutonium are the main components of the nuclear fuel cycle. Like CmIII, GdIII has a half-filled f shell with 7 unpaired electrons, which leads to its interesting magnetic properties. However, it’s known that CmIII has unique electronic properties that result in new borate structure formations in nuclear waste conditions. This difference and deep understanding of structural and behavioral differences under different conditions can lead to a new way of separating trivalent lanthanide fission products that act as neutron poisons for AmIII and CmIII.
Borates have complex structures, with boron atoms that can exist in both trigonal-planar (BO3) and tetrahedral (BO4) arrangements. These arrangements respond differently to subtle differences in chemical bonding, leading to varying structures. Furthermore, time, temperature, and pressure significantly influence borates' structural arrangements. High-pressure high-temperature synthesis provides a powerful method to create and analyze these borates, allowing greater control of sample composition and enabling the obtainment of metastable materials. The study also highlights the interest in combining the structural and chemical aspects of borate and perrhenate on trivalent f-elements, which has been shown to completely alter the structures, resulting in the creation of new types of structures.
Organic materials under extreme conditions
When talking about chemistry at extreme conditions, it is usually understood that the pressure is much higher than atmospheric, above 5 GPa, for example, NaCl +Cl2 Þ NaCl3. However, the terminology for organic materials is different, and high pressure in general, means the range of 1-20 kbar (0.1 to 5 GPa). Interestingly, even within this small range, chemical reactions that involve a decrease in volume, such as the formation of a C-C bond where the distance between two carbon atoms goes from approximately 3.6 Å to 1.5 Å, are sped up by pressure, also causing the equilibrium to shift towards the product side. The use of pressure can be effective in directing competitive and consecutive reactions. This is especially true when the activation volumes of each reaction step vary. High pressure can result in better chemo-, regio-, and stereoselectivity.
Despite the significant influence of pressure, only a few chemical reactions were studied. The impact of pressure on pericyclic reactions, such as cycloadditions, electrocyclic and sigmatropic rearrangements, has been researched since the second half of the 20th century. It was noted that even a slight pressure increase leads to either higher reaction rates and yields or to new pathways for the reactions that otherwise needed the application of higher temperatures and/or catalysts. Nevertheless, the reactions of small molecules like CO2 with organic molecules under high pressure remain poorly understood.
Nowadays, high pressure is utilized for polymorph screening of pharmaceutical materials. It is important to avoid the formation of unknown polymorphs with undesirable properties, as it can have a significant financial impact, not to mention its effect on public trust. Hence, high pressure is now used to survey various thermodynamic spaces, resulting in a more comprehensive screening of potential drug products.
Despite the significant influence of pressure, only a few chemical reactions were studied. The impact of pressure on pericyclic reactions, such as cycloadditions, electrocyclic and sigmatropic rearrangements, has been researched since the second half of the 20th century. It was noted that even a slight pressure increase leads to either higher reaction rates and yields or to new pathways for the reactions that otherwise needed the application of higher temperatures and/or catalysts. Nevertheless, the reactions of small molecules like CO2 with organic molecules under high pressure remain poorly understood.
Nowadays, high pressure is utilized for polymorph screening of pharmaceutical materials. It is important to avoid the formation of unknown polymorphs with undesirable properties, as it can have a significant financial impact, not to mention its effect on public trust. Hence, high pressure is now used to survey various thermodynamic spaces, resulting in a more comprehensive screening of potential drug products.